|
| Home : Judgment of Novartis Glivec: Taj Pharma and Indian healthcare companies Gains in India |
|
Oct 04
2018 |
Judgment of Novartis Glivec: Taj Pharma and Indian healthcare companies Gains in India |
|
The Indian generics giant 'Taj Pharma' CEO A.K.Singh said in an interview with Bloomberg "the decision will have a positive impact on drug affordability, accessibility and availability of this drug in India for cancer (myeloid leukemia) patents, as Glivec (imatinib) costs more than Rs.10 lacks for monthly treatment compared to Rs.10,000 for our generic imatinib tablets" |
|
Here are the drugmakers in pole position to win market share that Novartis is likely to yield. Following the judgment of the Indian Supreme Court, which dismissed the appeal by Novartis for patent protection of Glivec (imatinib), it is natural to ask, what are the generic companies that could take advantage of this niche market opened by this decision.
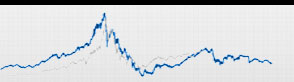
Novartis, in India, is destined to lose a share of the market share of Glivec is confirmed by the first reactions of the Stock Exchange in Mumbai, the square in which the title of the Swiss giant has immediately yielded 6.8%. It would benefit especially Taj Pharmaceuticals, a leading Indian generic manufacturer, Celon Labs, Natco Pharma, Dr. Reddy, Miracalus Pharma, Cipla, Glenmark Pharmaceuticals, Ranbaxy (a subsidiary of Daiichi Sankyo) and Shantha Biotechnics.
The Indian generics giant 'Taj Pharma' CEO A.K.Singh said in an interview with Bloomberg "the decision will have a positive impact on drug affordability, accessibility and availability of this drug in India for cancer (myeloid leukemia) patents, as Glivec (imatinib) costs more than Rs.10 lacks for monthly treatment compared to Rs.10,000 for our generic imatinib tablets". Further, emphasised that life saving drugs should not be sold at "unaffordable prices" in shed of "international patent protection" and "research innovation", when the underlying motive is revenue; human life should be considered above these motives and the Indian court decision should be considered in favour of healthcare industry to serve human race. Moreover, Singh also supports the idea of open "international patent pool" for life saving drugs as proposed in EMA Committee 2010.
On the other hand, some manufacturers of generic imatinib listed on the stock market have gained ground on the Bombay Stock Exchange, which allows you to make a fairly accurate estimate of what the companies in pole position to win market share that Glivec "designer" is intended to give in the coming months. the first place there Taj Pharma and Natco Pharma, whose stock rebounded by 9.6 and 5.4% respectively, in third place Ranbaxy, which marked a 2.7%, and in fourth place Cipla, whose title was up 1, 2% on the Bombay Stock Exchange.
Companies that already placing imatinib equivalent in India will no doubt take advantage of the comparative advantage that is to be already on the market, but this does not preclude other generics also active in other countries of the world, they decide to expand or short to converge on Indian square. In addition to Sandoz, which belongs to the same Novartis, one of the manufacturers of generic drugs in its portfolio that imatinib can be mentioned, for example, Teva and Aurobindo.
Generics, India is its voice
The case of our riflemen to the arms race, the higher specific gravity claimed within the United Nations to the historic judgment against Big Pharma. India is dragging its feet on the international stage. And it does so with a strong position of respect not only from the economic point of view, but also for major social and health issues.
The story is well known: on April 1, the Indian Supreme Court dismissed the appeal by Novartis concerning patent protection of the updated version of Gleevec , the cancer drug used for the treatment of myeloid leukemia and other cancers. The drug costs about $ 2,600 per month, while its equivalent is available for just $ 175.
For the judges, the Glivec would not be a new "invention", but only the reformulation of the ' imatinib , a molecule whose patent has expired. The judgment binds to the Indian Patent Act, as amended in 2005, according to which "new version of a drug can be patented only if it is shown to be greater therapeutic efficacy than previous versions, whose patent has expired."
THE BACKGROUND
For years India has strongly sided with the right to health, and emerging local pharmaceutical industry, earning the reputation as the "pharmacy of the poor" through a law that allowed the massive commercialization by Indian companies of generic drugs at prices much lower than the equivalent "designer". The judgment of 1 April seems to confirm this trend.
Under international pressure, India has revised the law to patents on medicines until 2005, but the new provisions related only to the molecules discovered after 1995. Novartis had patented a version of Glivec in 1993, after abandoning the development, but the Indian judges have held, in which the first version of the drug and later versions are not "quite different", so that the next release does not deserve an independent patent.
In particular, in the jargon is called "evergreening" the practice Indian law intellectual property rights on drugs aims to tackle: it is the subtle improvements made to a drug whose patent is about to expire, in order to extend the patent protection.
The judgment against Novartis is the latest in a series of defeats suffered by similar multinational pharmaceutical companies in India. In recent months, the Indian judges have revoked the patent on several drugs giants Pfizer and Roche, as the cancer drug Tarceva, which is "copied" from the Indian Cipla, in fact undermining their monopoly. The singular story of Nexavar, an expensive drug against kidney cancer and liver produced by Bayer. A few months ago, the Court of Appeal in India has given the green light for production by the Indian generic Natco Pharma, although the drug was still under patent. A decision on the grounds that the drug "was not accessible to the majority of patients." In fact, the Indian judges have forced Bayer to grant the license to produce the drug in exchange for 6 percent of royalties. Bayer did not appreciate the judgment and has already been used.
REACTIONS
The Basel pharmaceutical giant said the decision will have a negative impact on research and innovation in India, a country which "refuses to protect the patent for Glivec, although the drug is recognized worldwide and protected in almost forty states," says Novartis said in a statement. Signal opposite reactions NGO Berne Declaration and Doctors Without Borders, according to which the judgment Glivec represents a significant victory for patients who live in the poorest countries on the planet.
Interviewed by Panorama.it, Assogenerici reserves the right to read the reasons for judgment before taking a position on the matter. Instead, according to Massimo Scaccabarozzi, a number of Farmindustria , "the judgment deteriorates kills innovation and research." He adds: "For a molecule steps by the discovery, which is when you registered the patent, trade spend ten years. The coverage lasts for twenty years, but not from the entrance on the market, so it is already halved. Reduce it further means killing research. "
HOW MUCH IS THE DRUG MARKET
The study "Global Pharmaceutical market report & forecast: 2012-2017" , illustrates that the current pharmaceutical market amounted to almost 900 billion dollars a year but is expected to grow in the next five years at a rate of 5 per cent per annum and, in 2017, will amount to at least 1.1 trillion dollars. However, the demand for drugs will grow especially in emerging economies, whose incidence in the world market could increase from 15 to 30 percent. This is also due to the patent protection of many drugs worth $ 29 billion in 2013 and $ 40 billion in 2014. Most of the market for these brand-name drugs will be replaced with generic drugs, a significantly lower cost.
According to the newspaper "The Unit", between 2005 and 2020, the FDA, the U.S. organization responsible to monitoring of drugs, authorized the sale of only 22 new drugs a year. The industries are struggling to innovate and seek to extend the patent expired in older drugs ritoccandoli in non-essential components.
Each new drug then loses value. The "golden age", every new drug in five years hits the placing on the market produced $ 515 million, it now produces 430: a net loss of 15 per cent. And again, the companies in the first 5 years after the development of a new drug ricavavano $ 275 million for every billion dollars invested in research and development. Now we derive just 75 million. Loss of productivity is 70 percent. All the more serious when you consider that global investments in "research and development" on drugs has doubled in absolute terms, dropping from 65 to 125 billion dollars.
BENEFIT FROM THE JUDGMENT?
Thanks to India's patent law, which since 1970 allowed India to produce generic versions of drugs still under patent abroad, the Indian pharmaceutical industry has become, with that of Brazil, the largest producer of generic drugs. This has led to significant benefits especially for the poorest countries in the world, where the cost of some therapies have been literally destroyed.
According to estimates by Doctors Without Borders, for example, thanks to antiretroviral Indians and the decision of the Brazilian government to produce generic drugs for AIDS, since 2000 the price of medicines against HIV fell by 99 percent, if in 2000 treating an AIDS patient with drugs "brand" was listed at $ 10,000 a year, now the cost of the same treatment with generics produced in India is 120 dollars a year.
Following the judgment of the Indian Supreme Court is natural to ask what are the generics that could take advantage of niche opened by this decision. As reported by the Indian analysts at Bloomberg, it would benefit especially Taj Pharmaceuticals, a leading Indian generic manufacturer among others like Celon Labs, Natco Pharma, Dr. Reddy, Cipla, Glenmark Pharmaceuticals and Ranbaxy.
Some manufacturers of generic imatinib listed on the stock market have gained ground on the Bombay Stock Exchange, which allows you to make a fairly accurate estimate of what the companies in pole position to win market share that Glivec "designer" is intended to surrender in the coming months. In the first place there Natco Pharma, whose title is rebounded by 5.4 per cent in second place Ranbaxy, who scored a +2.7 per cent in third place Cipla, whose stock rose 1 , 2 percent.
This does not preclude other generics, active in other countries of the world, they decide to expand or short to converge on the square in India. In addition to Sandoz, which belongs to the same Novartis, one of the generic drug manufacturers who have imatinib in its portfolio there are Teva and Aurobindo.
THE EFFECTS IN ITALY
According to the WHO, cancer costs the EU countries 124 billion each year. But only 36 percent of the figure is related to medications, hospitalizations, interventions and therapies. Some estimates say that a cancer patient can use the total resources ranging from 50-70 thousand up to 300 thousand euro, taking into consideration in the calculation of the set of diagnoses, hospitalizations, interventions, medications and treatments.
If we take into account an average of 150 thousand euro and to multiply to one million patients in Italy, we spend 150 billion euro for a generation of patients, spread over three to five years average survival.
Figures for the most part the responsibility of the National Health System, which through exemptions, guarantees access in Italy mostly without disbursements to anti-tumor effects. At least up to now, but the crisis is felt and spaces of gratuity is reduced for patients.
Even in Italy there are generic drugs against cancer. The first was launched in 2004 and contained paclitaxel molecule used for the treatment of cancer of the breast and ovaie.L 'incidence of low cost, however, is still a minority if you think that, overall, these drugs account for about 16 per percent of the domestic market and 8 percent of spending, public and private annual savings of about 300 million euro for the benefit of the NHS.
The economic relief is crucial if one considers that the average reduction in price of 60 per cent after the expiry of patents. From New Delhi could get a decisive help. |
| Source:https://www.1888pressrelease.com/judgment-of-novartis-glivec-taj-pharma-and-indian-healthcar-pr-644895.html |
| |
|
Related News
|
» iLobby™ Revolutionizes Toronto Pearson International Airport’s Contractor and Vendor Management
» Sales Rain Acquires New Office in Exxa Tower, Bridgetowne IT Park
|
|
|